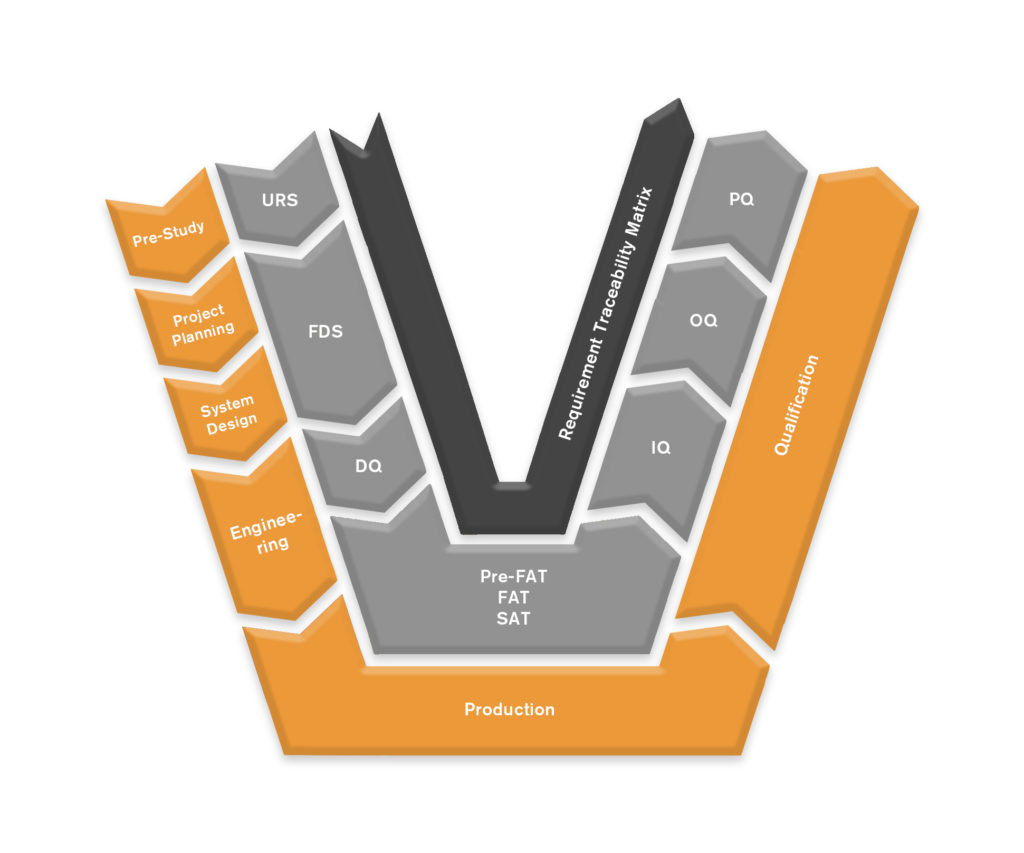
Our engineers and technicians deliver support services within qualification and validation of production processes. As a leading developer of automated production processes, with an in-depth understanding of GMP, ISO 13485 and GAMP5, the AP team is well suited to make a difference in your Medtech project.
We can join customer project teams as members for shorter or longer periods of time, or we can be assigned to deliver specific QA tasks.
- Developing technical documentation and test protocols (SAT, FAT plans)
- Risk analysis and CE-marking
- Review, analysis, and re-qualification of processes in order to improve and stabilize production
- Developing URS (User Requirements Specification) for customers investment projects
- Review of implemented QA control methods; validate or test?
Quality Assurance
- Design robustness
- DFM/DFA analysis
- Review of key processes and critical technologies
Test Documentation
- FAT – Plan, Perform and document
- SAT – Plan, Perform and document
- IQ & OQ – Plan, Perform and document
URS Support
- Identifying customer needs
- Understand and specify critical functions
- Authoring URS according to GAMP