Medical device manufacturers are always working to balance the demands of meeting government regulations, while reducing production costs, in an effort to produce the most reliable and safest medical devices
Automationspartner takes pride in being part of the value chain, putting safe medical devices and pharmaceutical products on the market. Improving patient safety, making sure your company earns recognition as an industry leader.
Doing so requires a structured way of working in projects with well-implemented procedures.
We have been ISO-certified since 2007.
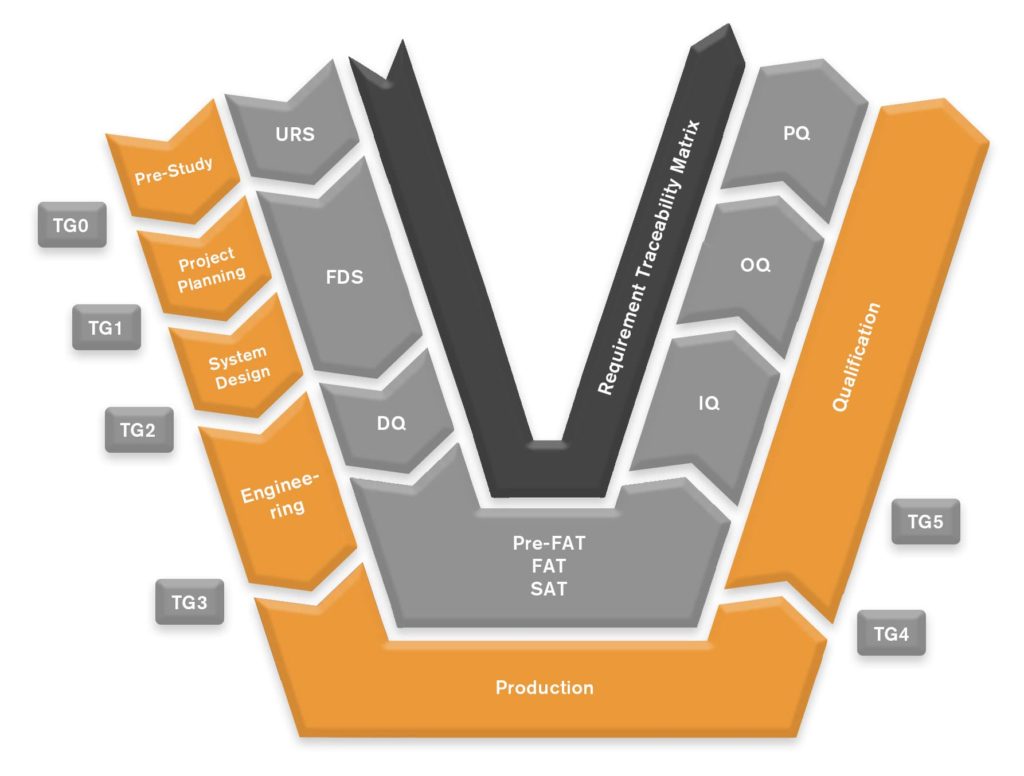
A rigid and proven process methodology is the foundation of every project. To closely manage the project in all its phases is important for a correct on time delivery. When in production, the project manager works closely with production. Every step is monitored, especially deviations. Deviations will always occur in large projects, but it’s what happens after they are discovered and how you learn from them that makes a difference.



